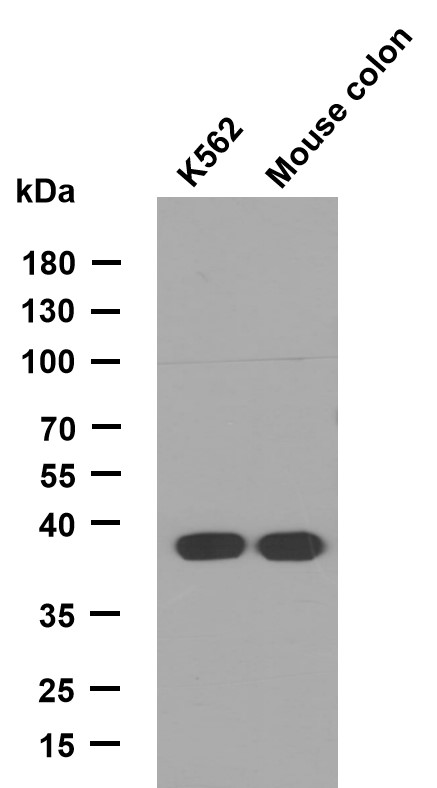
HSF1 (phospho Thr142) Polyclonal Antibody
- Catalog No.:YP1026
- Applications:IHC;IF;ELISA
- Reactivity:Human;Mouse
- Target:
- HSF1
- Fields:
- >>Legionellosis
- Gene Name:
- HSF1
- Protein Name:
- Heat shock factor protein 1
- Human Gene Id:
- 3297
- Human Swiss Prot No:
- Q00613
- Mouse Gene Id:
- 15499
- Mouse Swiss Prot No:
- P38532
- Immunogen:
- The antiserum was produced against synthesized peptide derived from human HSF1 around the phosphorylation site of Thr142. AA range:108-157
- Specificity:
- Phospho-HSF1 (T142) Polyclonal Antibody detects endogenous levels of HSF1 protein only when phosphorylated at T142.
- Formulation:
- Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
- Source:
- Polyclonal, Rabbit,IgG
- Dilution:
- IHC 1:100 - 1:300. ELISA: 1:20000.. IF 1:50-200
- Purification:
- The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
- Concentration:
- 1 mg/ml
- Storage Stability:
- -15°C to -25°C/1 year(Do not lower than -25°C)
- Other Name:
- HSF1;HSTF1;Heat shock factor protein 1;HSF 1;Heat shock transcription factor 1;HSTF 1
- Molecular Weight(Da):
- 31kD
- Background:
- heat shock transcription factor 1(HSF1) Homo sapiens The product of this gene is a transcription factor that is rapidly induced after temperature stress and binds heat shock promoter elements (HSE). This protein plays a role in the regulation of lifespan. Expression of this gene is repressed by phsphorylation, which promotes binding by heat shock protein 90. [provided by RefSeq, Aug 2016],
- Function:
- function:DNA-binding protein that specifically binds heat shock promoter elements (HSE) and activates transcription. In higher eukaryotes, HSF is unable to bind to the HSE unless the cells are heat shocked.,PTM:Phosphorylated on multiple serine residues, a subset of which are involved in stress-related regulation of transcription activation. Constitutive phosphorylation represses transcriptional activity at normal temperatures. Levels increase on specific residues heat-shock and enhance HSF1 transactivation activity. Phosphorylation on Ser-307 derepresses activation on heat-stress and in combination with Ser-303 phosphorylation appears to be involved in recovery after heat-stress. Phosphorylated on Ser-230 by CAMK2, in vitro. Cadmium also enhances phosphorylation at this site. Phosphorylation on Ser-303 is a prerequisite for HSF1 sumoylation. Phosphorylation on Ser-121 inhibits transacti
- Subcellular Location:
- Nucleus . Cytoplasm . Nucleus, nucleoplasm . Cytoplasm, perinuclear region . Cytoplasm, cytoskeleton, spindle pole . Cytoplasm, cytoskeleton, microtubule organizing center, centrosome . Chromosome, centromere, kinetochore . The monomeric form is cytoplasmic in unstressed cells (PubMed:8455624, PubMed:26159920). Predominantly nuclear protein in both unstressed and heat shocked cells (PubMed:10413683, PubMed:10359787). Translocates in the nucleus upon heat shock (PubMed:8455624). Nucleocytoplasmic shuttling protein (PubMed:26159920). Colocalizes with IER5 in the nucleus (PubMed:27354066). Colocalizes with BAG3 to the nucleus upon heat stress (PubMed:8455624, PubMed:26159920). Localizes in subnuclear granules called nuclear stress bodies (nSBs) upon heat shock (PubMed:11447121, PubMed:1151455
- Expression:
- Adipose tissue,Brain,Epithelium,Muscle,
- June 19-2018
- WESTERN IMMUNOBLOTTING PROTOCOL
- June 19-2018
- IMMUNOHISTOCHEMISTRY-PARAFFIN PROTOCOL
- June 19-2018
- IMMUNOFLUORESCENCE PROTOCOL
- September 08-2020
- FLOW-CYTOMEYRT-PROTOCOL
- May 20-2022
- Cell-Based ELISA│解您多样本WB检测之困扰
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-ACETYL-PROTEIN
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-PHOSPHO-PROTEIN
- July 13-2018
- Antibody-FAQs
- Products Images
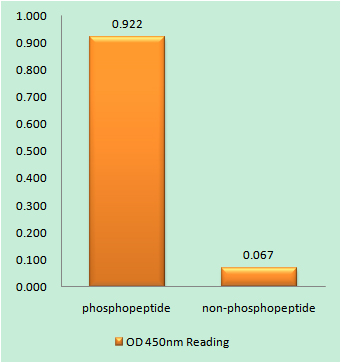
- Enzyme-Linked Immunosorbent Assay (Phospho-ELISA) for Immunogen Phosphopeptide (Phospho-left) and Non-Phosphopeptide (Phospho-right), using HSF1 (Phospho-Thr142) Antibody

- Immunohistochemistry analysis of paraffin-embedded human brain, using HSF1 (Phospho-Thr142) Antibody. The picture on the right is blocked with the phospho peptide.