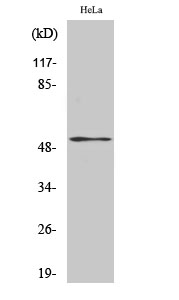
Phospho PTEN (S370) Cell-Based Colorimetric ELISA Kit
- Catalog No.:KA1642C
- Applications:ELISA
- Reactivity:Human;Mouse;Rat
- Gene Name:
- PTEN
- Human Gene Id:
- 5728
- Human Swiss Prot No:
- P60484
- Mouse Swiss Prot No:
- O08586
- Storage Stability:
- 2-8°C/6 months
- Other Name:
- Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (EC 3.1.3.16) (EC 3.1.3.48) (EC 3.1.3.67) (Mutated in multiple advanced cancers 1) (Phosphatase and tensin homolog)
- Detection Method:
- Colorimetric
- Background:
- catalytic activity:A phosphoprotein + H(2)O = a protein + phosphate.,catalytic activity:Phosphatidylinositol 3,4,5-trisphosphate + H(2)O = phosphatidylinositol 4,5-bisphosphate + phosphate.,catalytic activity:Protein tyrosine phosphate + H(2)O = protein tyrosine + phosphate.,cofactor:Magnesium.,disease:A microdeletion of chromosome 10q23 involving PTEN and BMPR1A is a cause of chromosome 10q23 deletion syndrome [MIM:612242]. This syndrome shows overlapping features of the following three disorders: Bannayan-Zonana syndrome, Cowden disease and juvenile polyposis syndrome.,disease:Defects in PTEN are a cause of Bannayan-Zonana syndrome (BZS) [MIM:153480]; also known as Ruvalcaba-Riley-Smith or Bannayan-Riley-Ruvalcaba syndrome (BRRS). In BZS there seems not to be an increased risk of malignancy. It has a partial clinical overlap with CD. BZS is characterized by the classic triad of macrocephaly, lipomatosis and pigmented macules of the gland penis.,disease:Defects in PTEN are a cause of Cowden disease (CD) [MIM:158350]; also known as Cowden syndrome (CS). CD is an autosomal dominant cancer predisposition syndrome associated with elevated risk for tumors of the breast, thyroid and skin. The predominant phenotype for CD is multiple hamartoma syndrome, in many organ systems including the breast (70% of CD patients), thyroid (40-60%), skin, CNS (40%), gastrointestinal tract. Affected individuals are at an increased risk of both breast and thyroid cancers. Trichilemmomas (benign tumors of the hair follicle infundibulum), and mucocutaneous papillomatosis (99%) are hallmarks of CD.,disease:Defects in PTEN are a cause of macrocephaly/autism syndrome [MIM:605309]. Patients have autism spectrum disorders and macrocephaly, with head circumferences ranging from +2.5 to +8 SD for age and sex (average head circumference +4.0 SD).,disease:Defects in PTEN are a cause of oligodendroglioma [MIM:137800]; also called oligodendroblastoma or familial glioma of brain. Oligodendroglioma is a usually benign neoplasm derived from and composed of oligodendrogliocytes in varying stages of differentiation. The majority are seen in adults in the white matter of the brain.,disease:Defects in PTEN are a cause of Proteus syndrome [MIM:176920]. Proteus syndrome is a hamartomatous disorder characterized by overgrowth of multiple tissues, connective tissue and epidermal naevi, and vascular malformations. These presentations are usually apparent at birth or soon after and continue to develop as the patient ages. It is named after the Greek god Proteus who, legend has it, could change his shape at will to avoid capture. Tumors, mostly benign but some malignant, have also been reported in Proteus syndrome, generally presenting by the age of 20 years and including papillary adenocarcinoma of the testis, meningioma, and cystadenoma of the ovaries.,disease:Defects in PTEN are a cause of squamous cell carcinoma of the head and neck (HNSCC) [MIM:275355].,disease:Defects in PTEN are a cause of susceptibility to endometrial cancer [MIM:608089].,disease:Defects in PTEN are a cause of VACTERL association with hydrocephalus [MIM:276950]; which includes also VATER association with hydrocephalus. VACTERL is an acronym for vertebral anomalies, anal atresia, congenital cardiac disease, tracheoesophageal fistula, renal anomalies, radial dysplasia, and other limb defects.,disease:Defects in PTEN are involved in prostate cancer [MIM:176807].,disease:Defects in PTEN are the cause of Lhermitte-Duclos disease (LDD) [MIM:158350]; also known as cerebelloparenchymal disorder VI. LDD is characterized by dysplastic gangliocytoma of the cerebellum which often results in cerebellar signs and seizures. LDD and CD seem to be the same entity, and are considered as hamartoma-neoplasia syndromes.,disease:Mutations of PTEN are found in a large number of cancers.,domain:The C2 domain binds phospholipid membranes in vitro in a Ca(2+)-independent manner; this binding is important for its tumor suppressor function.,function:Tumor suppressor. Acts as a dual-specificity protein phosphatase, dephosphorylating tyrosine-, serine- and threonine-phosphorylated proteins. Also acts as a lipid phosphatase, removing the phosphate in the D3 position of the inositol ring from phosphatidylinositol 3,4,5-trisphosphate, phosphatidylinositol 3,4-diphosphate, phosphatidylinositol 3-phosphate and inositol 1,3,4,5-tetrakisphosphate with order of substrate preference in vitro PtdIns(3,4,5)P3 > PtdIns(3,4)P2 > PtdIns3P > Ins(1,3,4,5)P4. The lipid phosphatase activity is critical for its tumor suppressor function. Antagonizes the PI3K-AKT/PKB signaling pathway by dephosphorylating phosphoinositides and thereby modulating cell cycle progression and cell survival. The unphosphorylated form cooperates with AIP1 to suppress AKT1 activation. Dephosphorylates tyrosine-phosphorylated focal adhesion kinase and inhibits cell migration and integrin-mediated cell spreading and focal adhesion formation. May be a negative regulator of insulin signaling and glucose metabolism in adipose tissue.,induction:Down-regulated by transforming growth factor beta (TGF-beta).,PTM:Phosphorylated in vitro by MAST1, MAST2 and MAST3. Phosphorylation results in an inhibited activity towards PIP3. Phosphorylation can both inhibit or promote PDZ-binding.,similarity:Contains 1 C2 tensin-type domain.,similarity:Contains 1 phosphatase tensin-type domain.,subunit:Monomer. The unphosphorylated form interacts with the second PDZ domain of AIP1 and with DLG1 and MAST2 in vitro. Interacts with MAGI2, MAGI3, MAST1 and MAST3, but neither with MAST4 nor with DLG5. Interaction with MAGI2 increases protein stability.,tissue specificity:Expressed at a relatively high level in all adult tissues, including heart, brain, placenta, lung, liver, muscle, kidney and pancreas.,
- Function:
- regulation of cyclin-dependent protein kinase activity, angiogenesis, blood vessel development, vasculature development, regulation of cell-matrix adhesion, negative regulation of cell-matrix adhesion, inositol metabolic process, protein amino acid dephosphorylation, phospholipid metabolic process, glycerophospholipid metabolic process, phosphorus metabolic process, phosphate metabolic process, apoptosis, induction of apoptosis, cell motion,negative regulation of cell adhesion, cell surface receptor linked signal transduction, enzyme linked receptor protein signaling pathway, transmembrane receptor protein tyrosine kinase signaling pathway, heart development, muscle organ development, aging, response to nutrient, behavior, learning or memory, memory, cell death, cell proliferation,negative regulation of cell proliferation, response to endogenous stimulus, response to hormone stimulus, re
- Subcellular Location:
- Cytoplasm . Nucleus . Nucleus, PML body . Monoubiquitinated form is nuclear. Nonubiquitinated form is cytoplasmic. Colocalized with PML and USP7 in PML nuclear bodies (PubMed:18716620). XIAP/BIRC4 promotes its nuclear localization (PubMed:19473982). .; [Isoform alpha]: Secreted . May be secreted via a classical signal peptide and reenter into cells with the help of a poly-Arg motif.
- Expression:
- Expressed at a relatively high level in all adult tissues, including heart, brain, placenta, lung, liver, muscle, kidney and pancreas.
- June 19-2018
- WESTERN IMMUNOBLOTTING PROTOCOL
- June 19-2018
- IMMUNOHISTOCHEMISTRY-PARAFFIN PROTOCOL
- June 19-2018
- IMMUNOFLUORESCENCE PROTOCOL
- September 08-2020
- FLOW-CYTOMEYRT-PROTOCOL
- May 20-2022
- Cell-Based ELISA│解您多样本WB检测之困扰
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-ACETYL-PROTEIN
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-PHOSPHO-PROTEIN
- July 13-2018
- Antibody-FAQs

.jpg)