Catalog: YT7124
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
TXD12
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
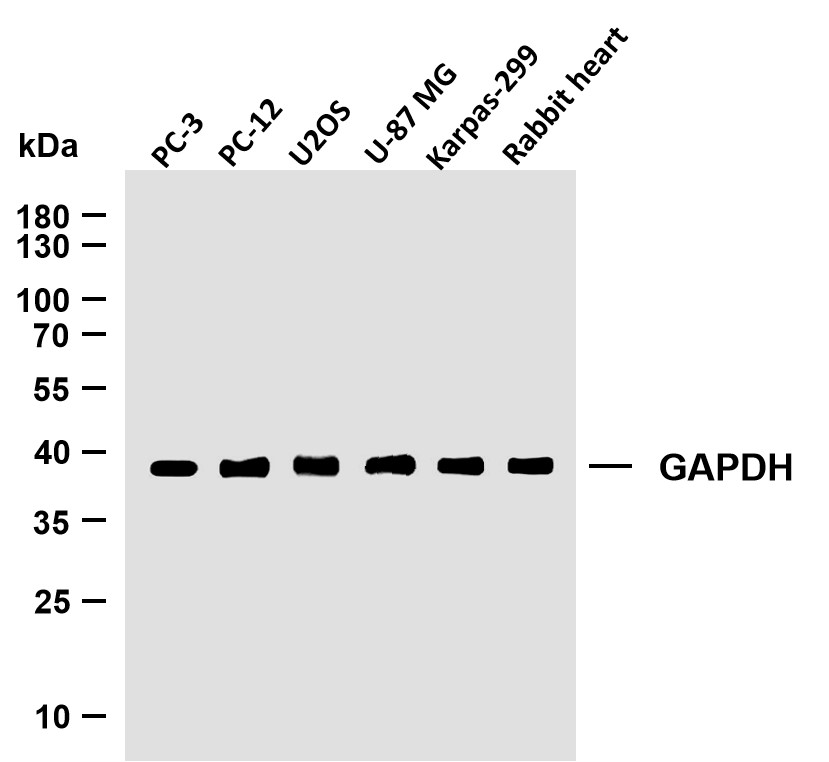
Applications
WB
MW
19kD (Calculated)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-2000
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
This antibody detects endogenous levels of TXD12 at Human/Mouse/Rat
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Calculated)
19kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human TXD12 AA range: 118-168
show all
Specificity:
This antibody detects endogenous levels of TXD12 at Human/Mouse/Rat
show all
Gene Name:
TXNDC12 TLP19 UNQ713/PRO1376
show all
Protein Name:
TXD12
show all
Database Link:
Background:
This gene encodes a member of the thioredoxin superfamily. Members of this family are characterized by a conserved active motif called the thioredoxin fold that catalyzes disulfide bond formation and isomerization. This protein localizes to the endoplasmic reticulum and has a single atypical active motif. The encoded protein is mainly involved in catalyzing native disulfide bond formation and displays activity similar to protein-disulfide isomerases. This protein may play a role in defense against endoplasmic reticulum stress. Alternate splicing results in both coding and non-coding variants. [provided by RefSeq, Mar 2012],
show all
Function:
Catalytic activity:2 glutathione + protein-disulfide = glutathione disulfide + protein-dithiol.,cofactor:FAD.,Function:Possesses significant protein thiol-disulfide oxidase activity.,Function:Probable oxidoreductase that acts as a caspase-independent mitochondrial effector of apoptotic cell death. Extramitochondrial AIF induces nuclear chromatin condensation and large scale DNA fragmentation (in vitro). Binds to DNA in a sequence-independent manner.,similarity:Belongs to the FAD-dependent oxidoreductase family.,similarity:Contains 1 thioredoxin domain.,subcellular location:Translocated to the nucleus upon induction of apoptosis.,subunit:Interacts with XIAP.,tissue specificity:Widely expressed.,
show all
Cellular Localization:
Endoplasmic reticulum lumen .
show all
Tissue Expression:
Widely expressed.
show all
Research Areas:
>>Glutathione metabolism ;
>>Metabolic pathways
>>Metabolic pathways
show all
Reference Citation({{totalcount}})
Catalog: YT7124
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}