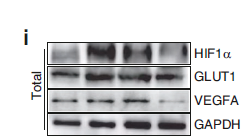
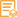Catalog: YT5686
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
ADAM10
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, ELISA
MW
70kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
WB 1:500-1:2000; IHC: 1:100-1:300; ELISA 1:10000; IF 1:50-200
Formulation
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
Specificity
CD156c Polyclonal Antibody detects endogenous levels of CD156c protein.
Purification
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
Concentration
1 mg/ml
MW(Observed)
70kD
Modification
Unmodified
Clonality
Polyclonal
Isotype
IgG
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from the Internal region of human CD156c.
show all
Specificity:
CD156c Polyclonal Antibody detects endogenous levels of CD156c protein.
show all
Gene Name:
ADAM10
show all
Protein Name:
Disintegrin and metalloproteinase domain-containing protein 10
show all
Other Name:
ADAM10 ;
KUZ ;
MADM ;
Disintegrin and metalloproteinase domain-containing protein 10 ;
ADAM 10 ;
CDw156 ;
Kuzbanian protein homolog ;
Mammalian disintegrin-metalloprotease ;
CD156c
KUZ ;
MADM ;
Disintegrin and metalloproteinase domain-containing protein 10 ;
ADAM 10 ;
CDw156 ;
Kuzbanian protein homolog ;
Mammalian disintegrin-metalloprotease ;
CD156c
show all
Background:
ADAM metallopeptidase domain 10(ADAM10) Homo sapiens Members of the ADAM family are cell surface proteins with a unique structure possessing both potential adhesion and protease domains. This gene encodes and ADAM family member that cleaves many proteins including TNF-alpha and E-cadherin. Alternate splicing results in multiple transcript variants encoding different proteins that may undergo similar processing. [provided by RefSeq, Feb 2016],
show all
Function:
Catalytic activity:Endopeptidase of broad specificity.,cofactor:Binds 1 zinc ion.,Domain:The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.,Function:Cleaves the membrane-bound precursor of TNF-alpha at '76-Ala-|-Val-77' to its mature soluble form. Responsible for the proteolytic release of several other cell-surface proteins, including heparin-binding epidermal growth-like factor, ephrin-A2 and for constitutive and regulated alpha-secretase cleavage of amyloid precursor protein (APP). Contributes to the normal cleavage of the cellular prion protein. Involved in the cleavage of the adhesion molecule L1 at the cell surface and in released membrane vesicles, suggesting a vesicle-based protease activity. Controls also the proteolytic processing of Notch and mediates lateral inhibition during neurogenesis.,induction:In osteoarthritis affected-cartilage.,PTM:The precursor is cleaved by a furin endopeptidase.,similarity:Contains 1 disintegrin domain.,similarity:Contains 1 peptidase M12B domain.,subcellular location:Is localized in the plasma membrane but is predominantly expressed in the Golgi apparatus and in released membrane vesicles derived likely from the Golgi.,subunit:Interacts with ephrin-A2.,tissue specificity:Expressed in spleen, lymph node, thymus, peripheral blood leukocyte, bone marrow, cartilage, chondrocytes and fetal liver.,
show all
Cellular Localization:
Cell membrane ; Single-pass type I membrane protein . Golgi apparatus membrane ; Single-pass type I membrane protein . Cytoplasmic vesicle, clathrin-coated vesicle . Cell projection, axon . Cell projection, dendrite . Cell junction, adherens junction . Cytoplasm . Is localized in the plasma membrane but is also expressed in the Golgi apparatus and in clathrin-coated vesicles derived likely from the Golgi (PubMed:12475894). During long term depression, it is recruited to the cell membrane by DLG1 (PubMed:23676497). The immature form is mainly located near cytoplasmic fibrillar structures, while the mature form is predominantly located at zonula adherens and the cell membrane (PubMed:30463011). The localization and clustering of mature ADAM10 to zonula adherens is regulated by AFDN, TSPAN33, PLEKHA7 and PDZD11 (PubMed:30463011). .
show all
Tissue Expression:
Expressed in the brain (at protein level) (PubMed:23676497). Expressed in spleen, lymph node, thymus, peripheral blood leukocyte, bone marrow, cartilage, chondrocytes and fetal liver (PubMed:11511685, PubMed:9016778).
show all
Research Areas:
>>Alzheimer disease ;
>>Epithelial cell signaling in Helicobacter pylori infection
>>Epithelial cell signaling in Helicobacter pylori infection
show all
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YT5686
Size
Price
Status
Qty.
200μL
$450.00
In stock
0
100μL
$280.00
In stock
0
40μL
$150.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}