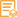Catalog: YM8683
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$340.00
3 weeks
0
40μL
$190.00
3 weeks
0
Add to cart


Collected


Collect
Main Information
Target
JNK1/2/3
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
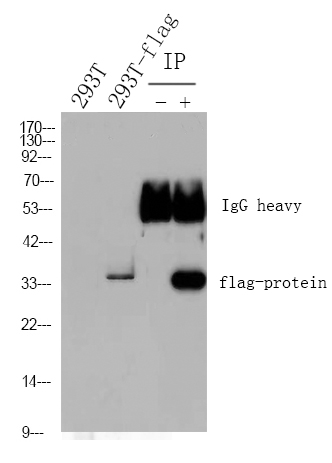
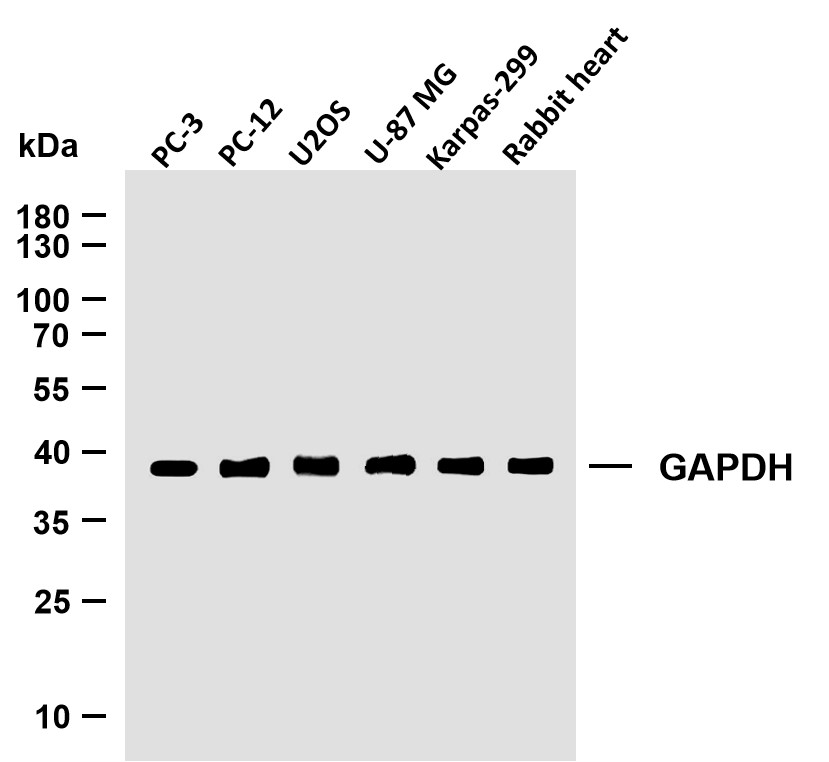
WB, IF, IP, ELISA
MW
48kD (Calculated)
46,54kD (Observed)
Conjugate/Modification
Phospho
Detailed Information
Recommended Dilution Ratio
WB 1:2000-1:10000; IF 1:200-1:1000; ELISA 1:5000-1:20000; IP 1:50-1:200;
Formulation
PBS, 50% glycerol, 0.05% Proclin 300, 0.05%BSA
Specificity
Phospho-JNK1/2/3 (T183/T183/T221) Antibody detects endogenous levels of JNK1/2/3 protein only when phosphorylated at T183/T183/T221.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):MMtPY
Purification
Protein A
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
MW(Calculated)
48kD
MW(Observed)
46,54kD
Modification
Phospho
Clonality
Monoclonal
Clone Number
PT0914R
Isotype
IgG,Kappa
Related Products
Antigen&Target Information
Specificity:
Phospho-JNK1/2/3 (T183/T183/T221) Antibody detects endogenous levels of JNK1/2/3 protein only when phosphorylated at T183/T183/T221.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):MMtPY
show all
Gene Name:
MAPK8/9/10
show all
Protein Name:
Mitogen-activated protein kinase 8/9/10
show all
Other Name:
MAPK8 ;
JNK1 ;
PRKM8 ;
SAPK1 ;
SAPK1C ;
Mitogen-activated protein kinase 8 ;
MAP kinase 8 ;
MAPK 8 ;
JNK-46 ;
Stress-activated protein kinase 1c ;
SAPK1c ;
Stress-activated protein kinase JNK1 ;
c-Jun N-terminal kinase 1 ;
MAPK9 ;
JNK2 ;
PRKM9 ;
SAPK1A ;
Mi
JNK1 ;
PRKM8 ;
SAPK1 ;
SAPK1C ;
Mitogen-activated protein kinase 8 ;
MAP kinase 8 ;
MAPK 8 ;
JNK-46 ;
Stress-activated protein kinase 1c ;
SAPK1c ;
Stress-activated protein kinase JNK1 ;
c-Jun N-terminal kinase 1 ;
MAPK9 ;
JNK2 ;
PRKM9 ;
SAPK1A ;
Mi
show all
Database Link:
Background:
The protein encoded by this gene is a member of the MAP kinase family. MAP kinases act as an integration point for multiple biochemical signals, and are involved in a wide variety of cellular processes such as proliferation, differentiation, transcription regulation and development. This kinase is activated by various cell stimuli, and targets specific transcription factors, and thus mediates immediate-early gene expression in response to cell stimuli. The activation of this kinase by tumor-necrosis factor alpha (TNF-alpha) is found to be required for TNF-alpha induced apoptosis. This kinase is also involved in UV radiation induced apoptosis, which is thought to be related to cytochrom c-mediated cell death pathway. Studies of the mouse counterpart of this gene suggested that this kinase play a key role in T cell proliferation, apoptosis and differentiation. Several alternatively spl
show all
Function:
Catalytic activity:ATP + a protein = ADP + a phosphoprotein.,cofactor:Magnesium.,Domain:The TXY motif contains the threonine and tyrosine residues whose phosphorylation activates the MAP kinases.,enzyme regulation:Activated by threonine and tyrosine phosphorylation by either of two dual specificity kinases, MAP2K4 and MAP2K7. Inhibited by dual specificity phosphatases, such as DUSP1.,Function:JNK1 isoforms display different binding patterns: beta-1 preferentially binds to c-Jun, whereas alpha-1, alpha-2, and beta-2 have a similar low level of binding to both c-Jun or ATF2. However, there is no correlation between binding and phosphorylation, which is achieved at about the same efficiency by all isoforms.,Function:Responds to activation by environmental stress and pro-inflammatory cytokines by phosphorylating a number of transcription factors, primarily components of AP-1 such as JUN, JDP2 and ATF2 and thus regulates AP-1 transcriptional activity. In T-cells, JNK1 and JNK2 are required for polarized differentiation of T-helper cells into Th1 cells (By similarity). Phosphorylates heat shock factor protein 4 (HSF4).,online information:C-Jun N-terminal kinases entry,PTM:Dually phosphorylated on Thr-183 and Tyr-185, which activates the enzyme.,similarity:Belongs to the protein kinase superfamily. CMGC Ser/Thr protein kinase family. MAP kinase subfamily.,similarity:Contains 1 protein kinase domain.,subunit:Binds to at least four scaffolding proteins, MAPK8IP1/JIP-1, MAPK8IP2/JIP-2, MAPK8IP3/JIP-3/JSAP1 and SPAG9/MAPK8IP4/JIP-4. These proteins also bind other components of the JNK signaling pathway. Interacts with TP53 and WWOX. Interacts with JAMP. Forms a complex with MAPK8IP1 and RGNEF (By similarity). Interacts with NFATC4.,
show all
Cellular Localization:
Cytoplasm . Nucleus . Cell junction, synapse . In the cortical neurons, predominantly cytoplasmic and associated with the Golgi apparatus and endosomal fraction. Increased neuronal activity increases phosphorylated form at synapses (By similarity). Colocalizes with POU5F1 in the nucleus. .
show all
Tissue Expression:
Brain,Epithelium,Fetal brain,Lung,Pooled,Testis,
show all
Research Areas:
>>Endocrine resistance ;
>>MAPK signaling pathway ;
>>ErbB signaling pathway ;
>>Ras signaling pathway ;
>>cAMP signaling pathway ;
>>FoxO signaling pathway ;
>>Sphingolipid signaling pathway ;
>>Mitophagy - animal ;
>>Autophagy - animal ;
>>Protein processing in endoplasmic reticulum ;
>>Apoptosis ;
>>Apoptosis - multiple species ;
>>Necroptosis ;
>>Wnt signaling pathway ;
>>Osteoclast differentiation ;
>>Focal adhesion ;
>>Tight junction ;
>>Toll-like receptor signaling pathway ;
>>NOD-like receptor signaling pathway ;
>>RIG-I-like receptor signaling pathway ;
>>C-type lectin receptor signaling pathway ;
>>IL-17 signaling pathway ;
>>Th1 and Th2 cell differentiation ;
>>Th17 cell differentiation ;
>>T cell receptor signaling pathway ;
>>Fc epsilon RI signaling pathway ;
>>TNF signaling pathway ;
>>Neurotrophin signaling pathway ;
>>Retrograde endocannabinoid signaling ;
>>Dopaminergic synapse ;
>>Inflammatory mediator regulation of TRP channels ;
>>Insulin signaling pathway ;
>>GnRH signaling pathway ;
>>Progesterone-mediated oocyte maturation ;
>>Prolactin signaling pathway ;
>>Adipocytokine signaling pathway ;
>>Relaxin signaling pathway ;
>>Type II diabetes mellitus ;
>>Insulin resistance ;
>>Non-alcoholic fatty liver disease ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Growth hormone synthesis, secretion and action ;
>>Alcoholic liver disease ;
>>Alzheimer disease ;
>>Parkinson disease ;
>>Huntington disease ;
>>Spinocerebellar ataxia ;
>>Prion disease ;
>>Pathways of neurodegeneration - multiple diseases ;
>>Epithelial cell signaling in Helicobacter pylori infection ;
>>Pathogenic Escherichia coli infection ;
>>Shigellosis ;
>>Salmonella infection ;
>>Pertussis ;
>>Yersinia infection ;
>>Chagas disease ;
>>Toxoplasmosis ;
>>Tuberculosis ;
>>Hepatitis B ;
>>Measles ;
>>Human T-cell leukemia virus 1 infection ;
>>Kaposi sarcoma-associated herpesvirus infection ;
>>Epstein-Barr virus infection ;
>>Human immunodeficiency virus 1 infection ;
>>Coronavirus disease - COVID-19 ;
>>Pathways in cancer ;
>>Chemical carcinogenesis - reactive oxygen species ;
>>Colorectal cancer ;
>>Pancreatic cancer ;
>>Choline metabolism in cancer ;
>>Diabetic cardiomyopathy ;
>>Lipid and atherosclerosis ;
>>Fluid shear stress and atherosclerosis
>>MAPK signaling pathway ;
>>ErbB signaling pathway ;
>>Ras signaling pathway ;
>>cAMP signaling pathway ;
>>FoxO signaling pathway ;
>>Sphingolipid signaling pathway ;
>>Mitophagy - animal ;
>>Autophagy - animal ;
>>Protein processing in endoplasmic reticulum ;
>>Apoptosis ;
>>Apoptosis - multiple species ;
>>Necroptosis ;
>>Wnt signaling pathway ;
>>Osteoclast differentiation ;
>>Focal adhesion ;
>>Tight junction ;
>>Toll-like receptor signaling pathway ;
>>NOD-like receptor signaling pathway ;
>>RIG-I-like receptor signaling pathway ;
>>C-type lectin receptor signaling pathway ;
>>IL-17 signaling pathway ;
>>Th1 and Th2 cell differentiation ;
>>Th17 cell differentiation ;
>>T cell receptor signaling pathway ;
>>Fc epsilon RI signaling pathway ;
>>TNF signaling pathway ;
>>Neurotrophin signaling pathway ;
>>Retrograde endocannabinoid signaling ;
>>Dopaminergic synapse ;
>>Inflammatory mediator regulation of TRP channels ;
>>Insulin signaling pathway ;
>>GnRH signaling pathway ;
>>Progesterone-mediated oocyte maturation ;
>>Prolactin signaling pathway ;
>>Adipocytokine signaling pathway ;
>>Relaxin signaling pathway ;
>>Type II diabetes mellitus ;
>>Insulin resistance ;
>>Non-alcoholic fatty liver disease ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Growth hormone synthesis, secretion and action ;
>>Alcoholic liver disease ;
>>Alzheimer disease ;
>>Parkinson disease ;
>>Huntington disease ;
>>Spinocerebellar ataxia ;
>>Prion disease ;
>>Pathways of neurodegeneration - multiple diseases ;
>>Epithelial cell signaling in Helicobacter pylori infection ;
>>Pathogenic Escherichia coli infection ;
>>Shigellosis ;
>>Salmonella infection ;
>>Pertussis ;
>>Yersinia infection ;
>>Chagas disease ;
>>Toxoplasmosis ;
>>Tuberculosis ;
>>Hepatitis B ;
>>Measles ;
>>Human T-cell leukemia virus 1 infection ;
>>Kaposi sarcoma-associated herpesvirus infection ;
>>Epstein-Barr virus infection ;
>>Human immunodeficiency virus 1 infection ;
>>Coronavirus disease - COVID-19 ;
>>Pathways in cancer ;
>>Chemical carcinogenesis - reactive oxygen species ;
>>Colorectal cancer ;
>>Pancreatic cancer ;
>>Choline metabolism in cancer ;
>>Diabetic cardiomyopathy ;
>>Lipid and atherosclerosis ;
>>Fluid shear stress and atherosclerosis
show all
Signaling Pathway
Cellular Processes >> Transport and catabolism >> Autophagy - animal
Cellular Processes >> Transport and catabolism >> Mitophagy - animal
Cellular Processes >> Cell growth and death >> Apoptosis
Cellular Processes >> Cell growth and death >> Apoptosis - multiple species
Cellular Processes >> Cell growth and death >> Necroptosis
Cellular Processes >> Cellular community - eukaryotes >> Focal adhesion
Cellular Processes >> Cellular community - eukaryotes >> Tight junction
Organismal Systems >> Immune system >> Toll-like receptor signaling pathway
Organismal Systems >> Immune system >> NOD-like receptor signaling pathway
Organismal Systems >> Immune system >> RIG-I-like receptor signaling pathway
Organismal Systems >> Immune system >> T cell receptor signaling pathway
Organismal Systems >> Immune system >> Th1 and Th2 cell differentiation
Organismal Systems >> Immune system >> Th17 cell differentiation
Organismal Systems >> Immune system >> IL-17 signaling pathway
Organismal Systems >> Immune system >> Fc epsilon RI signaling pathway
Organismal Systems >> Endocrine system >> Insulin signaling pathway
Organismal Systems >> Endocrine system >> Adipocytokine signaling pathway
Organismal Systems >> Endocrine system >> GnRH signaling pathway
Organismal Systems >> Endocrine system >> Prolactin signaling pathway
Organismal Systems >> Endocrine system >> Relaxin signaling pathway
Organismal Systems >> Endocrine system >> Growth hormone synthesis, secretion and action
Organismal Systems >> Nervous system >> Dopaminergic synapse
Organismal Systems >> Nervous system >> Neurotrophin signaling pathway
Organismal Systems >> Sensory system >> Inflammatory mediator regulation of TRP channels
Organismal Systems >> Development and regeneration >> Osteoclast differentiation
Human Diseases >> Cancer: overview >> Pathways in cancer
Human Diseases >> Cancer: specific types >> Colorectal cancer
Human Diseases >> Cancer: specific types >> Pancreatic cancer
Human Diseases >> Neurodegenerative disease >> Alzheimer disease
Human Diseases >> Neurodegenerative disease >> Parkinson disease
Human Diseases >> Neurodegenerative disease >> Huntington disease
Human Diseases >> Neurodegenerative disease >> Spinocerebellar ataxia
Human Diseases >> Neurodegenerative disease >> Prion disease
Human Diseases >> Neurodegenerative disease >> Pathways of neurodegeneration - multiple diseases
Environmental Information Processing >> Signal transduction >> MAPK signaling pathway
Environmental Information Processing >> Signal transduction >> ErbB signaling pathway
Environmental Information Processing >> Signal transduction >> Ras signaling pathway
Environmental Information Processing >> Signal transduction >> Wnt signaling pathway
Environmental Information Processing >> Signal transduction >> TNF signaling pathway
Environmental Information Processing >> Signal transduction >> FoxO signaling pathway
Environmental Information Processing >> Signal transduction >> Sphingolipid signaling pathway
Environmental Information Processing >> Signal transduction >> cAMP signaling pathway
Reference Citation({{totalcount}})
Catalog: YM8683
Size
Price
Status
Qty.
200μL
$600.00
3 weeks
0
100μL
$340.00
3 weeks
0
40μL
$190.00
3 weeks
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}