Catalog: YA0019
Size
Price
Status
Qty.
200μg
$600.00
3 weeks
0
100μg
$340.00
3 weeks
0
40μg
$190.00
3 weeks
0
Add to cart


Collected


Collect
Main Information
Target
CD59
Reactivity
Human
Applications
ELISA
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
ELISA 1:5000-100000
Formulation
Phosphate-buffered solution
Source
Camel, chimeric fusion of Nanobody (VHH) and mouse IgG1 Fc domain , recombinantly produced from 293F cell
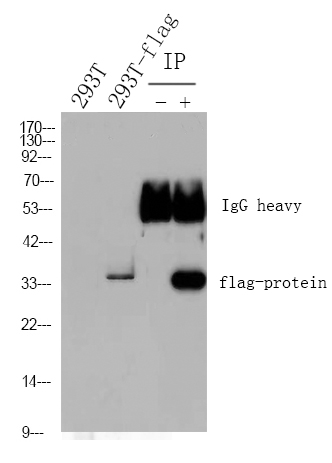
Specificity
This recombinant monoclonal antibody can detects endogenous levels of CD59 protein.
Purification
Recombinant Expression and Affinity purified
Storage
-15°C to -25°C/1 year(Avoid freeze / thaw cycles)
Concentration
Please check the information on the tube
Modification
Unmodified
Clone Number
PN0119
Related Products
Antigen&Target Information
Immunogen:
Purified recombinant Human CD59
show all
Specificity:
This recombinant monoclonal antibody can detects endogenous levels of CD59 protein.
show all
Gene Name:
CD59 MIC11 MIN1 MIN2 MIN3 MSK21
show all
Protein Name:
CD59 glycoprotein (1F5 antigen) (20 kDa homologous restriction factor) (HRF-20) (HRF20) (MAC-inhibitory protein) (MAC-IP) (MEM43 antigen) (Membrane attack complex inhibition factor) (MACIF) (Membrane inhibitor of reactive lysis) (MIRL) (Protectin) (CD antigen CD59)
show all
Other Name:
CD59 ;
MIC11 ;
MIN1 ;
MIN2 ;
MIN3 ;
MSK21 ;
CD59 glycoprotein ;
1F5 antigen ;
20 kDa homologous restriction factor ;
HRF-20 ;
HRF20 ;
MAC-inhibitory protein ;
MAC-IP ;
MEM43 antigen ;
Membrane attack complex inhibition factor ;
MACIF ;
Membrane inhibitor of reactive lysis ;
MIRL ;
Protectin ;
CD59 ;
CD59 nanobody ;
MIC11 ;
MIN1 ;
MIN2 ;
MIN3 ;
MSK21 ;
CD59 glycoprotein ;
1F5 antigen ;
20 kDa homologous restriction factor ;
HRF-20 ;
HRF20 ;
MAC-inhibitory protein ;
MAC-IP ;
MEM43 antigen ;
Membrane attack complex inhibition factor ;
MACIF ;
Membrane inhibitor of reactive lysis ;
MIRL ;
Protectin ;
CD59 ;
CD59 nanobody ;
show all
Background:
This gene encodes a cell surface glycoprotein that regulates complement-mediated cell lysis, and it is involved in lymphocyte signal transduction.This protein is a potent inhibitor of the complement membrane attack complex, whereby it binds complement C8 and/or C9 during the assembly ofThis complex, thereby inhibiting the incorporation of multiple copies of C9 into the complex, which is necessary for osmolytic pore formation.This protein also plays a role in signal transduction pathways in the activation of T cells. Mutations inThis gene cause CD59 deficiency, a disease resulting in hemolytic anemia and thrombosis, and which causes cerebral infarction. Multiple alternatively spliced transcript variants, which encode the same protein, have been identified forThis gene. [provided by RefSeq, Jul 2008]
show all
Function:
Disease:Defects in CD59 are the cause of CD59 deficiency [MIM:612300].,Potent inhibitor of the complement membrane attack complex (MAC) action. Acts by binding to the C8 and/or C9 complements of the assembling MAC, thereby preventing incorporation of the multiple copies of C9 required for complete formation of the osmolytic pore. This inhibitor appears to be species-specific. Involved in signal transduction for T-cell activation complexed to a protein tyrosine kinase.,The soluble form from urine retains its specific complement binding activity, but exhibits greatly reduced ability to inhibit MAC assembly on cell membranes.,online information:CD59 mutation db,PTM:Glycated. Glycation is found in diabetic subjects, but only at minimal levels in nondiabetic subjects. Glycated CD59 lacks MAC-inhibitory function and confers to vascular complications of diabetes.,PTM:N- and O-glycosylated. The N-glycosylation mainly consists of a family of biantennary complex-type structures with and without lactosamine extensions and outer arm fucose residues. Also significant amounts of triantennary complexes (22%). Variable sialylation also present in the Asn-43 oligosaccharide. The predominant O-glycans are mono-sialylated forms of the disaccharide, Gal-beta-1,3GalNAc, and their sites of attachment are probably on Thr-76 and Thr-77. The GPI-anchor of soluble urinary CD59 has no inositol-associated phospholipid, but is composed of seven different GPI-anchor variants of one or more monosaccharide units. Major variants contain sialic acid, mannose and glucosamine Sialic acid linked to an N-acetylhexosamine-galactose arm is present in two variants.,similarity:Contains 1 UPAR/Ly6 domain.,subcellular location:Soluble form found in a number of tissues.,subunit:Interacts with T-cell surface antigen CD2.,
show all
Cellular Localization:
Cell membrane; Lipid-anchor, GPI-anchor. Secreted. Soluble form found in a number of tissues.
show all
Tissue Expression:
Detected on umbilical veil endothelial cells (PubMed:162579). Detected in placenta (at protein level) (PubMed:169283). Detected on endothelial cells (PubMed:169283).
show all
Reference Citation({{totalcount}})
Catalog: YA0019
Size
Price
Status
Qty.
200μg
$600.00
3 weeks
0
100μg
$340.00
3 weeks
0
40μg
$190.00
3 weeks
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}