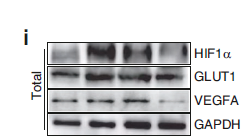
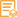Catalog: YM6810
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
40μL
$190.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
Mucin 1
Host Species
Mouse
Reactivity
Human
Applications
IHC, ELISA
MW
122kD (Calculated)
122kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
IHC 1:50-200; ELISA 1:500-5000
Formulation
PBS, 50% glycerol, 0.05% Proclin 300, 0.05%BSA
Specificity
The antibody can specifically recognize human MUC1 protein.
Purification
The antibody was affinity-purified from ascites by affinity-chromatography using specific immunogen.
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
MW(Calculated)
122kD
MW(Observed)
122kD
Modification
Unmodified
Clonality
Monoclonal
Clone Number
ABT100
Isotype
IgG1,Kappa
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human Epithelial Membrane Antigen(MUC1) AA range: 100-200
show all
Specificity:
The antibody can specifically recognize human MUC1 protein.
show all
Gene Name:
MUC1 PUM
show all
Protein Name:
Mucin-1 (MUC-1) (Breast carcinoma-associated antigen DF3) (Carcinoma-associated mucin) (Episialin) (H23AG) (Krebs von den Lungen-6) (KL-6) (PEMT) (Peanut-reactive urinary mucin) (PUM) (Polymorphic epithelial mucin) (PEM) (Tumor-associated epithelial membrane antigen) (EMA) (Tumor-associated mucin) (CD antigen CD227) [Cleaved into: Mucin-1 subunit alpha (MUC1-NT) (MUC1-alpha); Mucin-1 subunit beta (MUC1-beta) (MUC1-CT)], CA 15-3, CA15 3, CA 15.3 , CA15-3, CA15.3
show all
Other Name:
Mucin-1 ;
MUC-1 ;
Breast carcinoma-associated antigen DF3 ;
Carcinoma-associated mucin ;
Episialin ;
H23AG ;
Krebs von den Lungen-6 ;
KL-6 ;
PEMT ;
Peanut-reactive urinary mucin ;
PUM ;
Polymorphic epithelial mucin ;
PEM ;
Tumor-associated epithelial membrane antigen ;
EMA ;
Tumor-associated mucin ;
CD antigen CD227 ;
[Cleaved into: Mucin-1 subunit alpha ;
MUC1-NT ;
MUC1-alpha ;
Mucin-1 subunit beta ;
MUC1-beta ;
MUC1-CT ;
]
MUC-1 ;
Breast carcinoma-associated antigen DF3 ;
Carcinoma-associated mucin ;
Episialin ;
H23AG ;
Krebs von den Lungen-6 ;
KL-6 ;
PEMT ;
Peanut-reactive urinary mucin ;
PUM ;
Polymorphic epithelial mucin ;
PEM ;
Tumor-associated epithelial membrane antigen ;
EMA ;
Tumor-associated mucin ;
CD antigen CD227 ;
[Cleaved into: Mucin-1 subunit alpha ;
MUC1-NT ;
MUC1-alpha ;
Mucin-1 subunit beta ;
MUC1-beta ;
MUC1-CT ;
]
show all
Background:
This gene encodes a membrane-bound protein that is a member of the mucin family. Mucins are O-glycosylated proteins that play an essential role in forming protective mucous barriers on epithelial surfaces. These proteins also play a role in intracellular signaling. This protein is expressed on the apical surface of epithelial cells that line the mucosal surfaces of many different tissues including lung, breast stomach and pancreas. This protein is proteolytically cleaved into alpha and beta subunits that form a heterodimeric complex. The N-terminal alpha subunit functions in cell-adhesion and the C-terminal beta subunit is involved in cell signaling. Overexpression, aberrant intracellular localization, and changes in glycosylation of this protein have been associated with carcinomas. This gene is known to contain a highly polymorphic variable number tandem repeats (VNTR) domain. Alternate sp
show all
Function:
Alternative products:Additional isoforms seem to exist,Caution:O-glycosylation sites are annotated in first sequence repeat only. Residues at similar position are probably glycosylated in all repeats. Experimental sites were determined in a synthetic peptide glycosylated in vitro (PubMed:7744025, PubMed:9597769).,Caution:The N-terminal sequence has been shown (PubMed:11341784) to begin at position 24 or 28.,developmental stage:During fetal development, expressed at low levels in the colonic epithelium from 13 weeks of gestation.,Function:The alpha subunit has cell adhesive properties. Can act both as an adhesion and an anti-adhesion protein. May provide a protective layer on epithelial cells against bacterial and enzyme attack.,Function:The beta subunit contains a C-terminal domain which is involved in cell signaling, through phosphorylations and protein-protein interactions. Modulates signaling in ERK, Src and NF-kappaB pathways. In activated T-cells, influences directly or indirectly the Ras/MAPK pathway. Promotes tumor progression. Regulates P53-mediated transcription and determines cell fate in the genotoxic stress response. Binds, together with KLF4, the PE21 promoter element of P53 and represses P53 activity.,polymorphism:The number of repeats is highly polymorphic. It varies from 21 to 125 in the northern European population. The most frequent alleles contains 41 and 85 repeats. The tandemly repeated icosapeptide underlies polymorphism at three positions: PAPGSTAP[PAQT]AHGVTSAP[DT/ES]R, DT -> ES and the single replacements P -> A, P -> Q and P-> T. The most frequent replacement DT > ES occurs in up to 50% of the repeats.,PTM:Dual palmitoylation on cysteine residues in the CQC motif is required for recycling from endosomes back to the plasma membrane.,PTM:Highly glycosylated (N- and O-linked carbohydrates and sialic acid). O-glycosylated to a varying degree on serine and threonine residues within each tandem repeat, ranging from mono- to penta-glycosylation. The average density ranges from about 50% in human milk to over 90% in T47D breast cancer cells. Further sialylation occurs during recycling. Membrane-shed glycoproteins from kidney and breast cancer cells have preferentially sialyated core 1 structures, while secreted forms from the same tissues display mainly core 2 structures. The O-glycosylated content is overlapping in both these tissues with terminal fucose and galactose, 2- and 3-linked galactose, 3- and 3,6-linked GalNAc-ol and 4-linked GlcNAc predominating. Differentially O-glycosylated in breast carcinomas with 3,4-linked GlcNAc. N-glycosylation consists of high-mannose, acidic complex-type and hybrid glycans in the secreted form MUC1/SEC, and neutral complex-type in the transmembrane form, MUC1/TM.,PTM:Phosphorylated on tyrosines and serine residues in the C-terminal. Phosphorylation on tyrosines in the C-terminal increases the nuclear location of MUC1 and beta-catenin. Phosphorylation by PKC delta induces binding of MUC1 to beta-catenin/CTNNB1 and thus decreases the formation of the beta-catenin/E-cadherin complex. Src-mediated phosphorylation inhibits interaction with GSK3B. Src-and EGFR-mediated phosphorylation on Tyr-1229 increases binding to beta-catenin/CTNNB1. GSK3beta-mediated phosphorylation on Ser-1227 decreases this interaction but restores the formation of the beta-cadherin/E-cadherin complex. On T-cell receptor activation, phosphorylated by LCK. PDGFR-mediated phosphorylation increases nuclear colocalization of MUC1CT and CTNNB1.,PTM:Proteolytic cleavage in the SEA domain occurs in the endoplasmic reticulum by an autoproteolytic mechanism and requires the full-length SEA domain as well as requiring a Ser, Thr or Cys residue at the P + 1 site. Cleavage at this site also occurs on isoform MUC1/X but not on isoform MUC1/Y. Ectodomain shedding is mediated by ADAM17.,similarity:Contains 1 SEA domain.,subcellular location:Exclusively located in the apical domain of the plasma membrane of highly polarized epithelial cells. After endocytosis, internalized and recycled to the cell membrane. Located to microvilli and to the tips of long filopodial protusions.,subcellular location:On EGF and PDGFRB stimulation, transported to the nucleus through interaction with CTNNB1, a process which is stimulated by phosphorylation. On HRG stimulation, colocalizes with JUP/gamma-catenin at the nucleus.,subunit:The alpha subunit forms a tight, non-covalent heterodimeric complex with the proteolytically-released beta-subunit. Interaction, via the tandem repeat region, with domain 1 of ICAM1 is implicated in cell migration and metastases. Isoform 1 binds directly the SH2 domain of GRB2, and forms a MUC1/GRB2/SOS1 complex involved in RAS signaling. The cytoplasmic tail (MUC1CT) interacts with several proteins such as SRC, CTNNB1 and ERBs. Interaction with the SH2 domain of CSK decreases interaction with GSK3B. Interacts with CTNNB1/beta-catenin and JUP/gamma-catenin and promotes cell adhesion. Interaction with JUP/gamma-catenin is induced by heregulin. Binds PRKCD, ERBB2, ERBB3 and ERBB4. Heregulin (HRG) stimulates the interaction with ERBB2 and, to a much lesser extent, the interaction with ERBB3 and ERBB4. Interacts with P53 in response to DNA damage. Interacts with KLF4. Interacts with estrogen receptor alpha/ESR1, through its DNA-binding domain, and stimulates its transcription activity. Binds ADAM17.,tissue specificity:Expressed on the apical surface of epithelial cells, especially of airway passages, breast and uterus. Also expressed in activated and unactivated T-cells. Overexpressed in epithelial tumors, such as breast or ovarian cancer and also in non-epithelial tumor cells. Isoform 7 expressed in tumor cells only.,
show all
Cellular Localization:
Cytoplasmic, Membranous
show all
Reference Citation({{totalcount}})
Catalog: YM6810
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
40μL
$190.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}