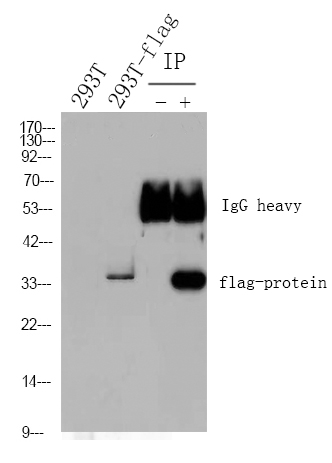
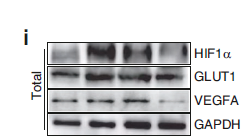
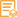Catalog: YM4925
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
40μL
$190.00
In stock
0
Add to cart


Collected


Collect
Main Information
Target
Collagen III
Host Species
Mouse
Reactivity
Human,
Applications
IHC, WB, IF, ELISA
MW
150kD (Calculated)
200kD (Observed)
Conjugate/Modification
Unmodified
Detailed Information
Recommended Dilution Ratio
IHC 1:200-1000; WB 1:500-2000; IF 1:100-500; ELISA 1:1000-5000
Formulation
PBS, 50% glycerol, 0.05% Proclin 300, 0.05%BSA
Specificity
This antibody detects endogenous levels of COL3A1 protein.
Purification
Protein G
Storage
-15°C to -25°C/1 year(Do not lower than -25°C)
MW(Calculated)
150kD
MW(Observed)
200kD
Modification
Unmodified
Clonality
Monoclonal
Clone Number
PT0118
Isotype
IgG1,Kappa
Related Products
Antigen&Target Information
Immunogen:
Synthesized peptide derived from human Collagen Type III AA range: 100-200
show all
Specificity:
This antibody detects endogenous levels of COL3A1 protein.
show all
Gene Name:
COL3A1
show all
Protein Name:
Collagen alpha-1(III) chain
show all
Background:
collagen type III alpha 1 chain(COL3A1) Homo sapiens This gene encodes the pro-alpha1 chains of type III collagen, a fibrillar collagen that is found in extensible connective tissues such as skin, lung, uterus, intestine and the vascular system, frequently in association with type I collagen. Mutations in this gene are associated with Ehlers-Danlos syndrome types IV, and with aortic and arterial aneurysms. Two transcripts, resulting from the use of alternate polyadenylation signals, have been identified for this gene. [provided by R. Dalgleish, Feb 2008],
Function:
Disease:Defects in COL3A1 are a cause of Ehlers-Danlos syndrome type 3 (EDS3) [MIM:130020]; also known as benign hypermobility syndrome. EDS is a connective tissue disorder characterized by hyperextensible skin, atrophic cutaneous scars due to tissue fragility and joint hyperlaxity. EDS3 is a form of Ehlers-Danlos syndrome characterized by marked joint hyperextensibility without skeletal deformity.,Disease:Defects in COL3A1 are a cause of susceptibility to aortic aneurysm abdominal (AAA) [MIM:100070]. AAA is a common multifactorial disorder characterized by permanent dilation of the abdominal aorta, usually due to degenerative changes in the aortic wall. Histologically, AAA is characterized by signs of chronic inflammation, destructive remodeling of the extracellular matrix, and depletion of vascular smooth muscle cells.,Disease:Defects in COL3A1 are the cause of Ehlers-Danlos syndrome type 4 (EDS4) [MIM:130050]. EDS is a connective tissue disorder characterized by hyperextensible skin, atrophic cutaneous scars due to tissue fragility and joint hyperlaxity. EDS4 is the most severe form of the disease. It is characterized by the joint and dermal manifestations as in other forms of the syndrome, characteristic facial features (acrogeria) in most patients, and by proneness to spontaneous rupture of bowel and large arteries. The vascular complications may affect all anatomical areas.,Function:Collagen type III occurs in most soft connective tissues along with type I collagen.,online information:Collagen type III alpha-1 chain mutations,online information:Type-III collagen entry,PTM:O-linked glycan consists of a Glc-Gal disaccharide bound to the oxygen atom of a post-translationally added hydroxyl group.,PTM:Proline residues at the third position of the tripeptide repeating unit (G-X-Y) are hydroxylated in some or all of the chains.,similarity:Belongs to the fibrillar collagen family.,similarity:Contains 1 VWFC domain.,subunit:Trimers of identical alpha 1(III) chains. The chains are linked to each other by interchain disulfide bonds. Trimers are also cross-linked via hydroxylysines.,
show all
Cellular Localization:
Cytoplasmic
show all
Tissue Expression:
Colon carcinoma,Liver,Placenta,Skin fibroblast,
show all
Research Areas:
>>Platelet activation ;
>>Relaxin signaling pathway ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Protein digestion and absorption ;
>>Amoebiasis ;
>>Diabetic cardiomyopathy
>>Relaxin signaling pathway ;
>>AGE-RAGE signaling pathway in diabetic complications ;
>>Protein digestion and absorption ;
>>Amoebiasis ;
>>Diabetic cardiomyopathy
show all
Signaling Pathway
Reference Citation({{totalcount}})
Catalog: YM4925
Size
Price
Status
Qty.
200μL
$600.00
In stock
0
100μL
$340.00
In stock
0
40μL
$190.00
In stock
0
Add to cart


Collected


Collect
Recently Viewed Products
Clear allPRODUCTS
CUSTOMIZED
ABOUT US
Toggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
Main Information
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
Product {{index}}/{{pcount}}
Prev
Next
{{pvTitle}}
Scroll wheel zooms the picture
{{pvDescr}}